December 7, 2023
Final FDA guidance establishes a risk-informed framework for assessing the credibility of computational modeling and simulation in medical device premarket submissions
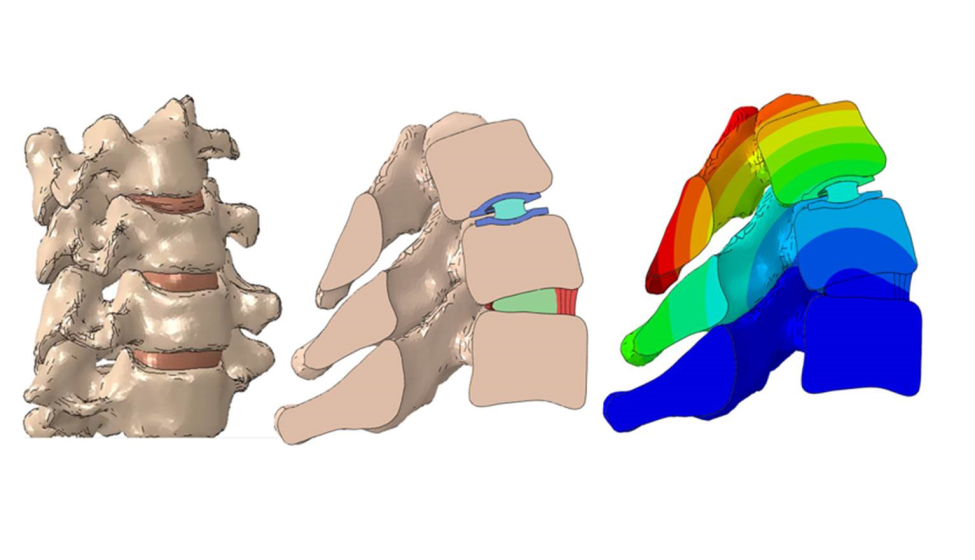
On Nov. 17, the Food and Drug Administration released the final guidance for assessing the credibility of computational modeling and simulation (CM&S) in medical device submissions. The guidance provides a greater level of clarity for the medical device industry as CM&S plays a growing role in supporting the development and regulatory submissions of new products through in silico evaluation and in silico clinical trials.
Framework for establishing the credibility of in silico assessment of medical devices
Medical device developers have increasingly turned to CM&S to virtually evaluate device designs, optimize benchtop testing, and perform in silico clinical trials. While credibility assessment frameworks have existed for some time (most notably in the form of the FDA consensus standard ASME V&V 40), they often rely on "traditional" sources of credibility evidence, particularly controlled benchtop experiments. As the applications for CM&S grow into more complex and physiologically realistic scenarios, such as in silico clinical trials and digital twin applications, FDA has recognized a need for a framework that incorporates evidence sources not covered by existing recognized industry standards.
In addition to stated goals related to streamlining the content in regulatory submissions and creating a common framework for planning and executing CM&S activities, the final guidance provides clarification on the expected steps required to establish levels of model credibility appropriate for the application of interest.
FDA outlines nine steps as part of the proposed generalized framework:
Step 1: State question of interest
Step 2: State context of use (COU)
Step 3: Assess model risk
Step 4: Identify credibility evidence to be collected
Step 5: State credibility factors
Step 6: Perform prospective adequacy assessment
Step 7: Generate credibility evidence
Step 8: Perform post-study adequacy assessment
Step 9: Prepare final Credibility Assessment Report
The framework provided in the guidance will assist device developers with navigating the complexities associated with CM&S in medical device development, most notably the highly variable nature of human physiology and disease.
This framework is particularly relevant as CM&S activities become more common throughout the total product lifecycle, moving beyond their traditional use in guiding preclinical activities and into burgeoning application areas of in silico clinical trials and patient-specific modeling associated with digital twins and pre-operative planning. Notably, the guidance builds on methods described in ASME V&V 40 for assessing how well the credibility activities establish overall model credibility for the chosen context of use.
Planning, executing, and documenting in silico models for regulatory submissions
By providing this generalized guidance framework, FDA is offering a streamlined process with the anticipated technical steps to establish the credibility of in silico models, potentially reducing uncertainty about regulatory acceptance for device submissions and enhancing the value of in silico predictions in support of device safety and efficacy.
The final guidance assists in creating sponsor/regulator alignment and facilitating efficient regulatory review by recommending regular pre-submission meetings to discuss CM&S plans and creating a documentation strategy for submitting results within the broader submission package.
For clarification on what the new industry guidance document covers, FDA added that CM&S refers to first principles-based (e.g., physics-based or mechanistic) computational models. This guidance document does not apply to statistical or data-driven (e.g., machine learning or artificial intelligence-based) models.
Given the diversity of existing CM&S applications and methods in life sciences, FDA welcomes online comments on the finalized guidance for assessing the credibility of CM&S evidence in medical device submissions. FDA is also conducting a webinar on Jan. 11, 2024, to review this final guidance.
What Can We Help You Solve?
Exponent has extensive experience conducting advanced CM&S to support regulatory submissions for medical devices. Our regulatory and technical experts help you develop computational models to characterize device performance, assess interactions with patient tissues and operational environments, and design relevant benchtop experiments to provide insight into model, test, and clinical results.
Medical Device Evaluation
Sophisticated medical device evaluations for a vast array of applications.

Medical Device Design & Development Support
Crucial medical device design and development analyses to empower your decision-making.

Regulatory Compliance for Medical Products
Experienced regulatory support for medical devices, pharmaceuticals, and combination products.
Multidisciplinary Expertise to Support the Life Sciences Industry
Support for next-gen medical devices, wearable technologies, and pharmaceutical products.
![Mechanical Modeling & Simulation [ME]](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-154962957.jpg?itok=ACTZQkIk)
Mechanical Modeling & Simulation
Predict and optimize mechanical performance, or discover the root cause of field failure with advanced mechanical modeling and simulation.
MRI Compatibility
MRI compatibility testing for medical devices to help ensure product safety and regulatory compliance.