How does Exponent leverage our unique multidisciplinary approach to help understand your real-world performance and provide feedback on the product lifecycle?
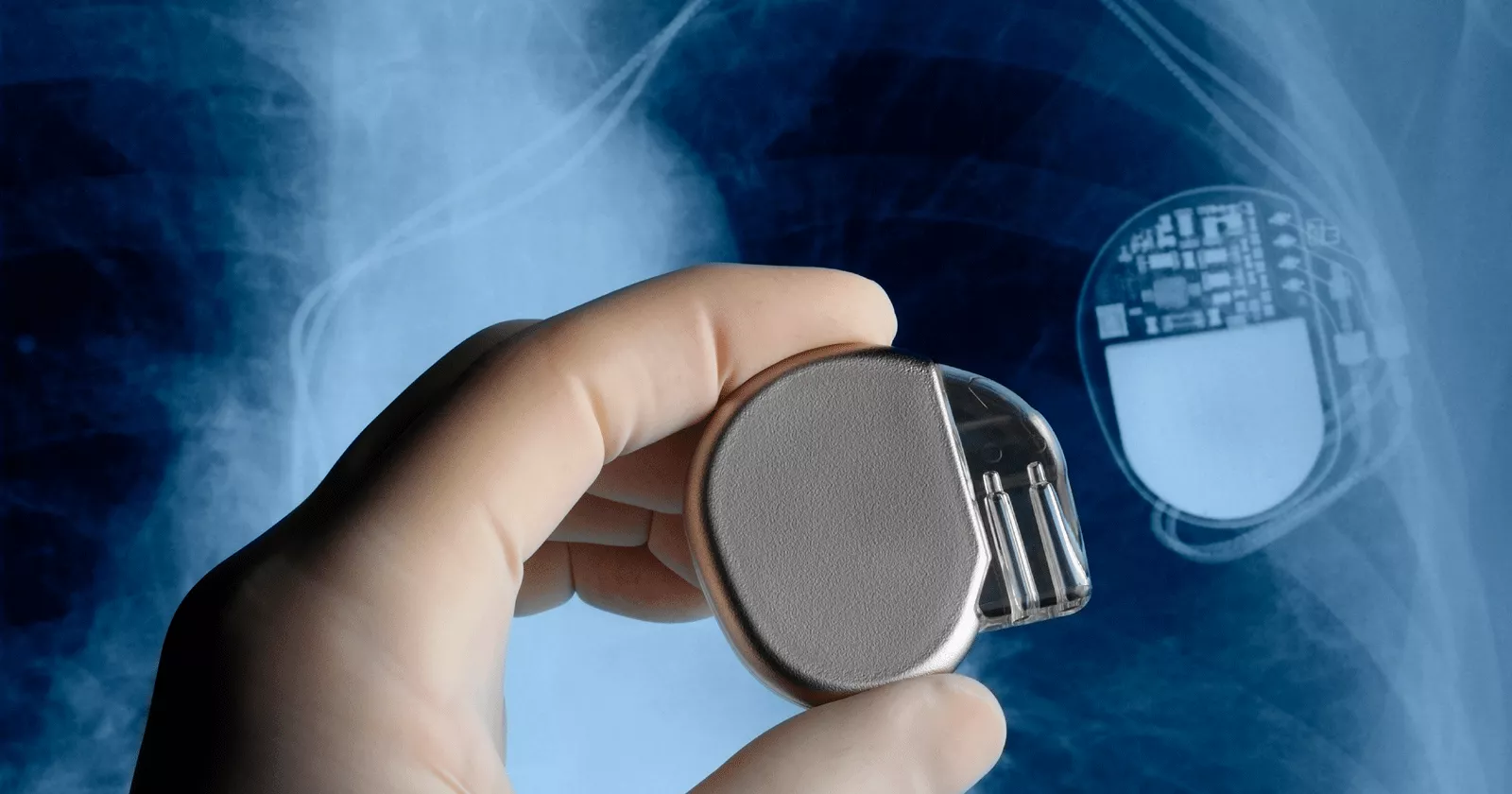
Exponent has extensive expertise in creating and managing international multicenter programs to collect and analyze components from explanted orthopedic, spine, and cardiovascular implants, including total joint replacements, artificial discs, surgical tools, pacemakers, implantable cardioverter-defibrillators, and stents.
In addition to explants, Exponent has created and managed programs to collect and analyze non-implantable medical devices such as surgical tools, therapeutic delivery systems, and combination products (e.g., autoinjectors). With a team of multidisciplinary experts highly trained in failure analysis, we have the experience to identify the cause when a failure occurs.
We are well-versed in the Food and Drug Administration's expectations for clinical studies and postmarket surveillance. Both require attention to retrieval analysis procedures that support a manufacturer's complaint handling process.
With accredited ISO 17025 (A2LA Certificate 2561.01, 2561.03) facilities for retrieval protocols based on ASTM F561, "Standard Practice for retrieval and analysis of medical devices and associated tissues and fluids," and ISO 12891, "Retrieval and analysis of surgical implants," Exponent's staff and laboratories are well positioned to serve the global community.
We have participated in the development of the standards and use them to create custom protocols to support appropriate collection, shipping, and analysis of retrieved devices, tissues, and non-implantable medical devices. Our team employs validated analysis techniques to assess wear and damage modes; evaluate fracture surfaces and material degradation; and quantify wear, deformation, and penetration of biomaterials. The results are compiled into reports suitable for direct submission to regulatory agencies.
Services
Exponent offers the following retrieved medical device and tissue analysis services:
• Development of customized retrieval protocols using ASTM F561 and ISO 12891 to meet regulatory requirements, complaint handling procedures, and total product lifecycle needs
• Clinical site training to support proper implementation of retrieval protocols
• Customized design of retrieval kits (including instructions and clinical data forms) compliant with international shipping regulations
• Selection of appropriate analysis techniques based on device material, anatomical location, and function
• Evaluation of retrievals composed of traditional (UHMWPE on CoCr) bearing materials, alternative (metal and ceramic) bearing materials, or novel bearings
• Assessment of wear patterns, failure mechanisms, fractures, corrosion, degradation, penetration, and deformation
• Examination of fluid and tissue surrounding the implant, including histological, trace metal, and particle analyses
• Assessment of user complaints for non-implantable devices, including autoinjectors
• Chemical and electrical evaluation of batteries in pacemakers and implantable cardioverter-defibrillators and associated leads
• Correlation of retrieved damage modes and mechanisms to in vitro tested devices
• Report preparation suitable for internal complaint handling and direct submission to regulatory bodies
• Response to FDA deficiency letters and other regulatory inquiries
Experts
Our global and comprehensive expertise across industries gives us a deep understanding of current challenges, best industry practices, and the implications of emerging technologies.

Corporate Vice President, Office Director and Principal

![Medical Device Design & Development Support [BES]](/sites/default/files/styles/fifty_node/public/media/images/GettyImages-1273852738.jpg.webp?itok=agkpoZT_)





