April 16, 2024
The agency has published new draft guidance to support manufacturer compliance with special controls
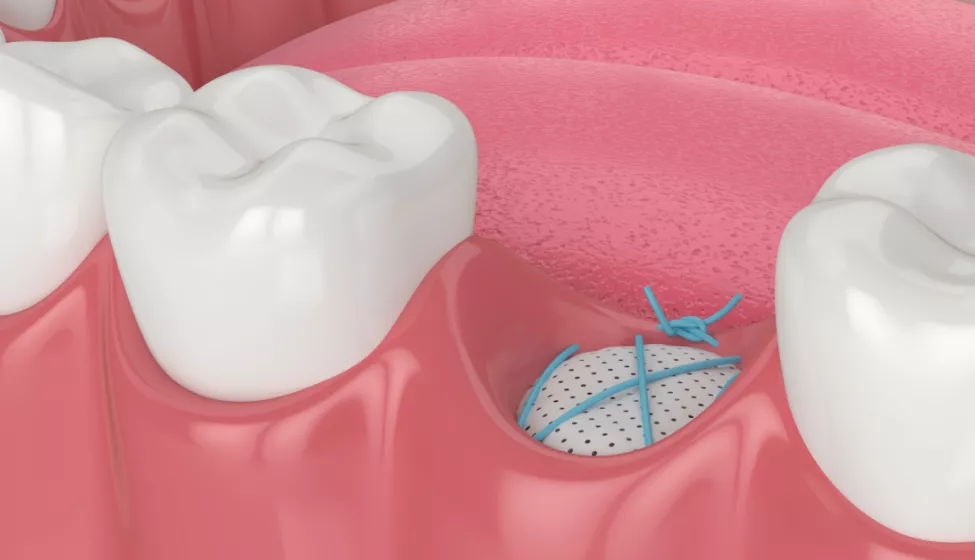
On March 29, the Food and Drug Administration issued draft guidance outlining recommendations for the use of animal studies to evaluate in vivo performance of dental bone grafting material devices. The recommendations are intended to help manufacturers comply with special controls for these material devices, promote consistency in the review process, and ensure the safety and effectiveness of these devices.
The agency's guidance covers devices that are materials "intended to fill, augment, or reconstruct periodontal or bony defects of the oral and maxillofacial region" and offers recommendations for study planning, animal model selection, monitoring, evaluation, test facility considerations, and reporting requirements for premarket submissions.
FDA has opened a 60-day public comment period through May 28 in the Federal Register of the proposed rule, or electronically, via regulations.gov referencing Docket No. FDA-2023-N-3392. FDA will consider all submitted feedback and suggestions before finalizing its guidance. When finalized, manufacturers will benefit from incorporating strategies for optimal in vivo study design and techniques for microarchitecture and tissue ingrowth assessment.
Specific guidance for animal studies and premarket submissions
In this new draft guidance document, FDA recommends conducting animal studies for inclusion in premarket submissions for dental bone grafting material devices. The document cautions against "extrapolating the in vivo behavior of a proposed bone grafting material device from the known in vivo behavior of a predicate bone grafting material device."
The agency further recommends:
- A minimum of three evaluation time points, allowing for "assessment in trends for graft resorption and new bone formation over time, as well as any inflammatory reactions."
- A minimum of three animals per treatment per evaluation time point.
- Canine or porcine, rather than rodent, animal models, due to the resemblance to human dentoalveolar architecture and ability to place sufficient amounts of graft material.
The guidance recommends micro-computed tomography (microCT) for each animal at each evaluation time point to provide additional three-dimensional detail and quantitative information on device microarchitecture and tissue ingrowth. Histologic analysis can provide a qualitative analysis of tissue types and confirm the presence of bone and residual implant throughout the defect over time.
FDA's guidance also includes recommendations for biocompatibility assessments, including circumstances where manufacturers may combine device performance evaluations and portions of biocompatibility assessments in a single in vivo study, per ISO 10993-6 Biological evaluation of medical devices — Part 6: Tests for local effects after implantation.
The guidance in the FDA's draft document is intended to complement the agency's General Considerations for Animal Studies Intended to Evaluate Medical Devices and, in some cases, supersede the controls outlined for dental bone grafting material devices in the agency's Class II special controls guidelines. The full draft guidance can be downloaded from the FDA website here.
What Can We Help You Solve?
Exponent's multidisciplinary medical device consultants have extensive experience providing safety and regulatory compliance support, including retrieved device and tissue analysis, micro-CT imaging, biocompatibility and biological risk assessments, preclinical and clinical study design, collaboration with in vivo testing facilities, MRI compatibility testing, pre- and postmarket assessments, and preparing 510(k) submissions.

Medical Device Design & Development Support
Crucial medical device design and development analyses to empower your decision-making.

Biocompatibility & Biological Risk Assessment
Address biocompatibility challenges throughout the medical-device product lifecycle.

Multidisciplinary Expertise to Support the Life Sciences Industry
Support for next-gen medical devices, wearable technologies, and pharmaceutical products.

Medical Device Evaluation
Sophisticated medical device evaluations for a vast array of applications.

Biomedical Engineering Laboratories
Expert biomedical laboratory services, delivering evidence-based answers for your most complex challenges.
Retrieved Device & Tissue Analysis
Retrieved device and tissue analysis to support medical device development and post-market surveillance.