February 16, 2023

Alternative methods could offer equally accurate results at a reduced computational burden
Medical device manufacturers could be facing new challenges in evaluating the safety of patients with implanted medical devices in magnetic resonance imaging (MRI) environments. The recent FDA guidance recommends that device manufacturers take into account how the benchtop test environment differs from the human body by putting results in the context of clinical use. Therefore, conventional testing methodologies outlined in ASTM F2182, which uses soft-tissue mimics and exposure conditions that are not clinically relevant, are no longer sufficient for evaluating patient safety due to radiofrequency (RF) induced heating.
This is particularly true for devices implanted in bone, because the medium described in ASTM F2182 is a soft-tissue mimic. For these devices, the ASTM F2182 measurement of heating is inaccurate due to how differently the RF electric field interacts with bone compared to soft tissue. Using this measurement to predict clinical heating produces results that could over- or underestimate patient risks.
Accounting for the heterogeneity of thermal and electromagnetic properties of both hard (e.g., bone) and soft (e.g., muscle) tissues in the body presents a unique challenge that requires substantial know-how and computational resources. The question is how to minimize the burden of analysis while still generating sufficient results to satisfy regulators and protect patients.
The heating standard
MRI has been a valuable clinical imaging tool for more than 35 years. It offers a viable alternative to imaging modalities that use ionizing radiation, which can damage or even destroy cells.
MRI has potential hazards as well, since the RF energy used during scans can heat tissue and cause burns. The Food and Drug Administration received 1,568 reports of adverse events related to MRI systems between 2008 and 2017, with most injuries (59% of analyzed results) caused by RF-induced heating of tissue around implanted medical devices.
To address potential MRI-related risks, FDA, other regulatory bodies, and international standards committees have recommended that device manufacturers test their implanted medical devices for MRI compatibility using ASTM F2182, the so-called "heating" standard, since 2002.
Rapidly changing regulatory environment
The recommendation that heating results derived from the traditional test environment should be evaluated in the context of the clinical environment first appears in the 2019 version of ASTM F2182. The 2021 FDA guidance on testing and labeling medical devices for safety in the MRI environment provides further details surrounding this concept, indicating that "to ensure that a clinically relevant worst-case heating scenario is assessed" multiple variables should be considered including patient anatomy, tissue properties, medical device characteristics, and location within the RF field and within or on the patient.
For medical devices implanted in bone, what is the most effective testing technique for device manufacturers to obtain FDA clearance and bring their products to market?
FDA and other regulatory bodies discussed this recommendation extensively at the International Society for Magnetic Resonance in Medicine 2022 Workshop on MR Safety: From Physics & Physiology to Policies & Practice. It may become part of ASTM F2182 once the community reaches consensus.
Until then, adhering to the standard test method may be sufficient for characterizing RF-induced heating for implanted devices in soft tissue, such as heart valves, since the ASTM F2182 test medium adequately mimics soft tissue. For devices implanted in bone, however, the current standard test method does not identify an appropriate test medium, severely limiting the use of the standard for such devices.
This raises the question: For medical devices implanted in bone, what is the most effective testing technique for device manufacturers to obtain FDA clearance and bring their products to market?
More is more?
Changes to standards and regulatory guidance language on this topic have been evolving for the past two years and have only become more complex. Over time, this has resulted in a significant increase in "burden of analysis," which accounts for the time, cost, and labor required by a particular testing method.
However, Exponent's research shows other approaches may be more efficient and equally effective.
In "Variability in the Analysis Burden for Evaluating Radiofrequency Induced Heating of Implanted Medical Devices" presented at the Biomedical Engineering Society 2022 Annual Meeting in San Antonio, Texas, our biomedical engineering experts compared the consensus "gold standard" test method for an implanted passive hip device to four alternative methods. These four alternative test methods evaluate RF heating by combining techniques from both ASTM F2182 and ISO/TS 10974:2018 to predict in vivo temperature rise. In this way, our team was able to evaluate how the test environment differs from the human body by putting results in the context of clinical use.
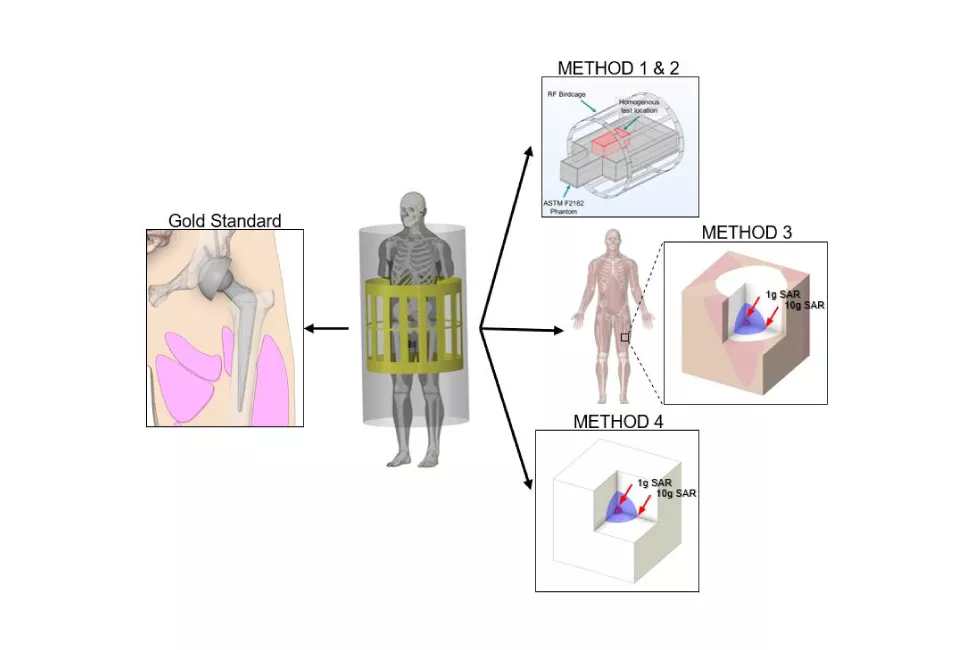
"Gold Standard" per ISO/TS 10974:2018: Computational evaluation using virtual implantation in human body model; electromagnetic and heat transfer physics simulated to determine temperature change.
Method 1: Exposure Scaling: Heating in phantom; electric field in test location matches in vivo estimates.
Method 2: Temperature Scaling: Heating in phantom; electric field in test location is arbitrary (with temperature rise scaled based on the ratio of in vitro to in vivo electric field exposure).
Method 3: Point Source Human Body Model (hybrid approach): Heating in human body model; rate of heating identified in phantom per method 1; electric field in test location matches in vivo estimates.
Method 4: Point Source Idealized (hybrid approach): Comparable to method 3 but assumes that the tissue surrounding the device is homogeneous rather than using human body model.
Our findings show that while all test methods were equally accurate at predicting RF-induced heating, they varied significantly in terms of analysis burden. For example, Method 1 required less computational time by a factor of one to two orders of magnitude compared to the "gold standard." This novel approach for predicting RF-induced heating in implanted passive hip devices has the potential to be useful not only for devices implanted in bone but for a wide range of devices used in all tissue types, including vascular stents deployed in soft tissues.
Exponent's research in this area is ongoing, with publications planned. These approaches have the potential to help device manufacturers bring their products to market more quickly and efficiently while improving the predictability of in vivo heating and maximizing patient safety.
What Can We Help You Solve?
Exponent provides experienced MRI compatibility testing for medical devices to help ensure that MRI risks are mitigated and regulatory requirements are met.
![Computational Modeling [TS]](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-182174791.jpg.webp?itok=kmskqW9J)
Computational Modeling; Thermal Sciences
A less expensive and faster alternative to thermal or flow experiments.
![Medical Devices, Implants & Surgical Tools [MCE]](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-1182458826.jpg.webp?itok=z7lVBn-v)
Medical Devices
Design verification, usability studies, electrical medical equipment configuration, and laboratory testing.
![[MCE] [BES] Medical Device Development Support - a tray of surgical tools](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-1296782647.jpg.webp?itok=N2clVZFq)
Materials for Medical Devices, Implants & Surgical Tools
Unique multidisciplinary breakthrough insights for medical tools and devices.
MRI Compatibility
MRI compatibility testing for medical devices to help ensure product safety and regulatory compliance.