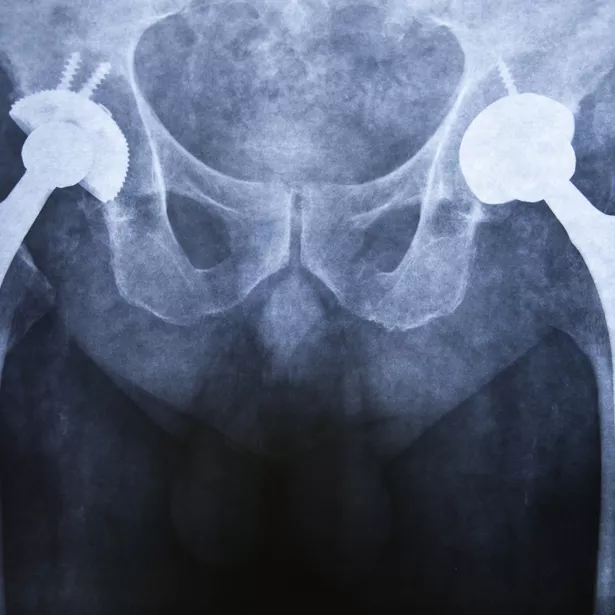
How does Exponent's multidisciplinary approach provide an accurate understanding of global regulatory challenges for medical devices and surgical implants?
Medical devices such as joint replacements, heart valves, drug delivery systems, battery casing, pacemakers, dental implants, and stents comprise a combination of biomaterials with different biocompatibility characteristics that can affect the human body in various ways.
Medical devices have expanded to include a wide variety of components containing polymeric, biologic, metallic, ceramic, and composite materials. As medical devices continue to advance in their capabilities and include more combinations of materials, regulatory frameworks are requiring biocompatibility as part of the functional requirements of these devices.
![Medical Devices, Implants & Surgical Tools [MCE]](/sites/default/files/styles/fifty_media_large/public/media/images/GettyImages-1182458826.jpg.webp?itok=_7QtJR9E)
What are biomedical materials, and how can they promote healing after surgery?
The concept of biocompatibility has expanded from identifying whether a product is inert, meaning non-toxic, non-antigenic, or non-mutagenic, to classifying materials as bioactive based on their ability to encourage positive healing responses by actively interacting with the tissue.
![Finite Element Analysis for Medical Devices & Biomaterials [BES]](/sites/default/files/styles/fifty_media_large/public/media/images/GettyImages-1223150089.jpg.webp?itok=MAmc519g)
Bioactive materials promote the formation of normal healthy tissue, as well as the integration of the device into adjacent tissue.
The new generation of implantable and tissue-engineered medical devices control biological interactions using pharmacological agents, bioactive coatings, or nano-enabled materials to improve safety and efficacy.
Our teams include experts in health sciences, mechanical engineering, materials and corrosion engineering, electrical engineering, biomedical engineering, pharmacology, and data science. Working together, we help you understand the various complexities of medical devices and implants.
Exponent offers biocompatibility testing plans, regulatory strategy advice, and biological safety evaluations customized to your medical device product. Our health scientists regularly conduct toxicological risk to complement ISO 1099318 chemical characterization testing.
![Medical Devices, Implants & Surgical Tools [PSMC]](/sites/default/files/styles/fifty_media_large/public/media/images/GettyImages-592647720.jpg.webp?itok=xlYXDZ0q)
How does Exponent evaluate a medical device's potential performance in real-life simulations?
We can characterize cells and tissues to the mechanical environment through assaying for matrix protein accumulation and real-time quantitative RT-PCR to determine gene expression and can provide real-time physiologic measures using fluorescent intra- and extracellular probes. We can also characterize protein changes in response to the mechanical environment through ELISAs (enzyme-linked immunosorbent assays), measurement of enzyme activity, and biochemical assays.
Our teams use 2D and 3D technology to characterize cellular responses to soluble factor gradients, cell migration assays, and autocrine/paracrine interactions, and can incorporate epifluorescent microscopy techniques, including confocal microscopy.
Exponent provides rigorous assessments for all types of medical devices.
Biocompatibility
Joint replacements
Hip implants
Knee implants
Heart valves
Stents
Dental devices
Cosmetic implants
Surgical tools
Surface devices
Wearable electronic devices
Battery casings
Capabilities
Whether seeking regulatory approval for a new medical device or evaluating the biocompatibility of an existing product with proposed changes, Exponent has the expertise and experience to help medical innovators throughout the entire product lifecycle.
Experts
Our global and comprehensive expertise across industries gives us a deep understanding of current challenges, best industry practices, and the implications of emerging technologies.


![Medical Devices [EECS]](/sites/default/files/styles/hero_purple/public/media/images/GettyImages-523306516.jpg.webp?itok=l6xPSsZ1)