February 13, 2026
Surgical planning leads AR/VR use, pushing manufacturers to focus on accuracy, appropriate use, training, and workflow integration
On Dec. 5, the United States Food and Drug Administration's Digital Health Center of Excellence updated its public list of medical devices that incorporate augmented reality (AR) or virtual reality (VR). The list now includes 104 entries, a 167% jump from the 39 entries FDA published in December 2022. This expansion reflects rapid growth in the use of extended-reality technologies in regulated clinical applications. FDA notes this list is not comprehensive, built largely from terms appearing in public decision summaries, but it provides a signal for where AR/VR is already established in regulated clinical workflows and where regulators and developers are likely to focus next.
AR/VR tools for surgical use surge ahead
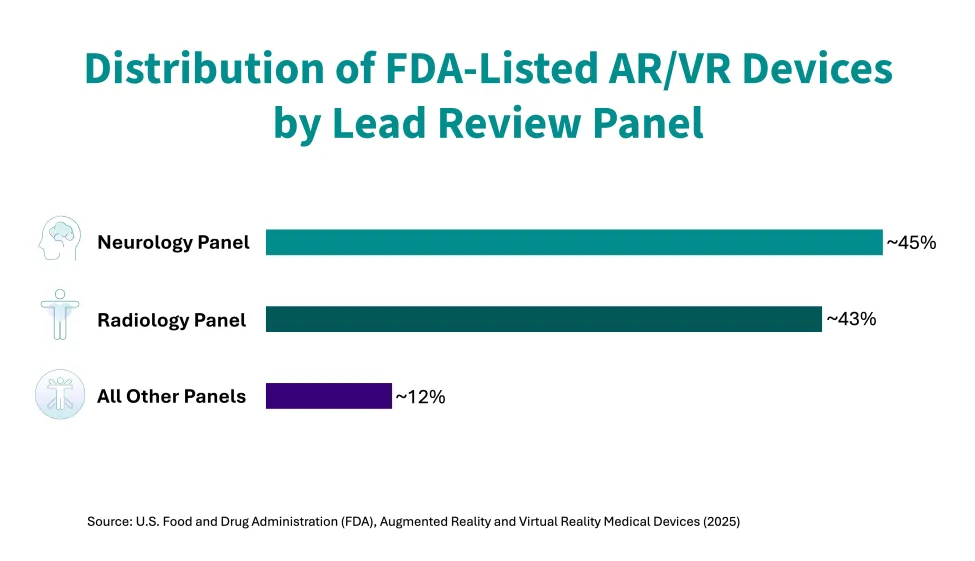
A clear theme in the updated list is the prominence of surgical planning, navigation, and visualization tools, particularly in orthopedics, neurosurgery, and radiology. Most listed devices were reviewed through neurology or radiology panels, and nearly three-quarters fall under just a few product codes tied to stereotactic and radiologic systems.
Notably, most devices currently on the list were cleared through the 510(k) pathway, along with two De Novo authorizations. This indicates that manufacturers are positioning AR/VR devices mostly as extensions of existing, legally marketed technologies, rather than as novel devices requiring more extensive FDA review.
Human factors risks shift to clinical judgment
As AR/VR systems become embedded in clinical decision making, FDA suggests that critical human factors concerns extend beyond comfort or interface usability. Instead, they increasingly focus on how the technology shapes clinical judgment under time pressure — evaluating not only whether users can operate the system but whether they can recognize when the system may be unreliable.
Key human factors considerations include overreliance on system cues, misalignment or latency that could distort perceived anatomy or guidance, whether users can recognize and recover from errors or system disconnects with sufficient time, and the burden of training and workflow integration in real clinical settings. These issues highlight FDA's emphasis on carefully weighing both the benefits and risks of AR/VR devices during medical use. Recent Class II recalls involving AR/VR-enabled surgical systems — often tied to software anomalies or image misalignment — underscore the importance of robust change control and postmarket risk management.
Pediatrics and home use raise concerns
The updated FDA list also includes therapeutic AR/VR devices intended for pediatric or home use, such as vision-related therapies. FDA has previously cautioned that long-term effects of immersive technologies may be less understood in pediatric populations, shifting human factors priorities toward understanding physical and cognitive developmental outcomes, comfort, symptom awareness, clear instructions, and reliable use outside clinical settings.
Is Virtual Reality Safe for Children?
What does expanded AR/VR use mean for manufacturers?
FDA's expanding list recognizes that AR/VR is no longer confined to demonstrations or training pilots. As AR/VR devices become embedded in clinical decision making, scrutiny is shifting from basic usability toward how these systems may affect judgment, including overreliance, misalignment, latency, and error recognition in real-world workflows. Manufacturers can plan early for software and hardware changes by developing predetermined change control plans, relying less on training or labeling for primary risk mitigations, and addressing pediatric and home-use risks proactively as FDA expectations continue to evolve despite limited AR/VR-specific guidance.
What Can We Help You Solve?
Exponent brings broad experience supporting the development and commercialization of medical devices, wearables, and electronics, with AR/VR-relevant expertise spanning human factors, cybersecurity, optics, batteries, systems engineering, materials, data analytics, and regulatory compliance.

Regulatory Compliance for Medical Products
Experienced regulatory support for medical devices, pharmaceuticals, and combination products.

Medical Technology Assessment Program
Rapid, expert assessments of medical devices and technologies, surgical implants, and wearable medical technologies.
![Medical Devices, Implants & Surgical Tools [MCE]](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-1182458826.jpg.webp?itok=z7lVBn-v)
Medical Devices
Design verification, usability studies, electrical medical equipment configuration, and laboratory testing.
![User Research & Testing [HF]](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-1338374024.jpg.webp?itok=bBXBoCIg)
Medical Device Usability Testing
Extensive human factors evaluations for medical device manufacturers in developing and navigating regulatory roadmaps.

Biosensor Integration & Biosignal Processing for UX Research
Leverage valuable psychophysiological and biosignal user data to enhance product design.

Medical Device Evaluation
Sophisticated medical device evaluations for a vast array of applications.





